-
ADMET in drug discovery and development
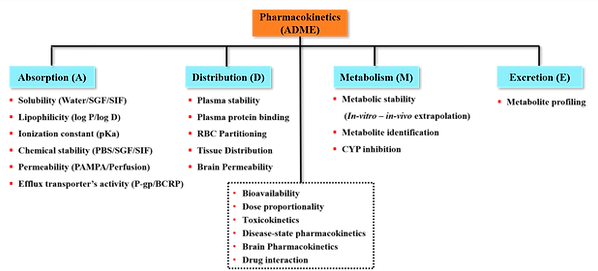
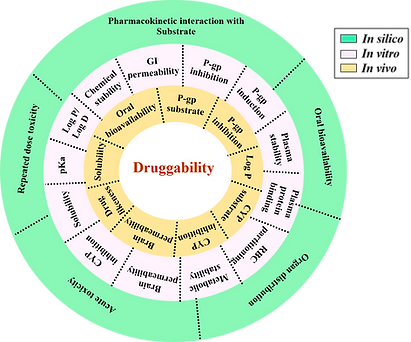
Currently, a major hurdle in drug discovery and development is the poor pharmacokinetic behavior of new chemical entities (NCEs). Early evaluation of ADME/PK properties is essential to assess clinical viability and to guide rational decisions on structural modifications or formulation strategies. We have established a robust and integrated platform comprising in vitro, ex vivo, in situ, and in vivo assays to comprehensively assess the druggability of NCEs in alignment with their intended therapeutic applications. As part of the drug development pipeline, we also possess expertise in conducting in vivo toxicological and safety pharmacology studies to support preclinical evaluation.
Our investigations aim to address the following key questions:
-
What factors contribute to the poor bioavailability of the molecule?
-
Does the molecule have any attributes indicative of druggability?
-
CYP regulation in disease state (Breast cancer)
Arachidonic acid metabolism via the Cytochrome P450 (CYP) epoxygenase pathway generates epoxyeicosatrienoic acids (EETs), which have been reported to promote tumorigenesis. While the COX and LOX pathways are well-characterized and widely targeted in drug discovery (NSAIDs and leukotriene antagonists), the role of the CYP epoxygenase pathway remains underexplored in the context of cancer. Our research aims to identify selective and potent CYP2J2 inhibitors from plant-derived natural products and to propose a tentative signaling mechanism by which CYP2J2 inhibition alters EET biosynthesis in triple-negative breast cancer (TNBC). This work is positioned within the framework of targeted cancer therapeutics to address tumor recurrence and drug resistance. We have established both in vitro and in vivo assay systems to elucidate the impact of CYP2J2 inhibition on cancer progression. Our investigations aim to address the following key questions :
-
What is the molecular mechanism of action of CYP2J2 inhibitors?
-
Can CYP2J2 be validated as a viable therapeutic target in cancer?

-
CYP regulation in disease states (Drug induced liver injury)
Cytochrome P450 2E1 (CYP2E1) plays a crucial role in hepatic drug metabolism but is also implicated in drug-induced liver injury (DILI). Although its basal activity is low, various physiological and dietary factors can induce CYP2E1 overexpression, leading to excessive production of reactive oxygen species (ROS), lipid peroxidation, and oxidative stress. This contributes to hepatocellular damage and the bioactivation of toxicants and pro-carcinogens. Despite its clinical significance, CYP2E1 remains an underexplored target for therapeutic intervention. Moreover, the mechanistic crosstalk between CYP2E1 and NF-κB signaling during oxidative stress in DILI is poorly understood. Investigating this axis and the involvement of nuclear receptors may offer novel insights for developing targeted hepatoprotective strategies. We have established in vitro and in vivo assay systems to evaluate CYP2E1 inhibition and developed preclinical DILI models to elucidate the hepatoprotective effects of CYP2E1 inhibitor. Our investigations aim to address the following key questions:
-
Does the role of a CYP2E1 inhibitor differ in DILI depending on CYP2E1 substrate vs. non-substrate?
-
What are the receptors (PXR/CAR/AhR) involved in CYP2E1/NF-κB axis?
Xenobiotics/Disease conditions

-
Selected publications



